Dossier: Innovative animal-free research in Baden-Württemberg
Mini-organs and multi-organ chips - where lab mice may soon retire
Farewell to animal testing? A closer look at Baden-Württemberg's research landscape reveals how life sciences researchers are pioneering innovative methods to replace animal experiments, minimise the number of animals used and refine the experimental procedures and conditions under which animals are kept. Replace, reduce, refine – these are the guiding principles of the 3Rs principle. The researchers are developing cutting-edge models and establishing a robust 3R network, which not only shapes the future of research but also improves the quality of scientific outcomes. This transformation is funded by the Baden-Württemberg Ministry of Science, Research and the Arts (MWK) with a substantial investment of 6.9 million euros.
Animal experiments remain a highly controversial topic and have increasingly been replaced by alternative methods, including in vitro and in silico approaches. Despite these advancements, it is estimated that approximately twelve million animal experiments are still conducted annually in Europe. Many of these are performed primarily because they are mandated by regulations for the approval of new pharmaceuticals.
 In many laboratories in Baden-Württemberg, researchers are already endeavouring to improve animal welfare by developing new technologies that supplement or eliminate the need for animal testing. The rabbit test for testing fever-inducing substances will finally be abolished in 2025 and replaced by new models. © Fiona Heyl, Freiburg
In many laboratories in Baden-Württemberg, researchers are already endeavouring to improve animal welfare by developing new technologies that supplement or eliminate the need for animal testing. The rabbit test for testing fever-inducing substances will finally be abolished in 2025 and replaced by new models. © Fiona Heyl, FreiburgIn 2022, a total of 361,043 animals were used or euthanised for scientific purposes in Baden-Württemberg. This has fallen from 393,760 in 2021 and dropped significantly from 533,685 in 2018.
Animal testing has historically been a cornerstone of medical research, playing a crucial role in the development of life-saving medications, including vaccines, insulin and antibiotics.
Before a drug can be placed on the market, it must undergo extensive preclinical testing, often involving animals, to evaluate its safety and efficacy. The authorisation process for such experiments is strictly regulated and ensures that animal testing is conducted only when absolutely necessary. This has led to a hybrid approach, which still involves traditional animal testing. However, advancements in technology are increasingly introducing innovative alternatives for addressing specific research questions, such as artificial organoids or organ-on-chip (OoC) models. These cutting-edge technologies offer more precise and ethical ways to evaluate drug safety and efficacy.
Humans are not nude 70 kg mice
Therapeutic suitability in the preclinical phase has traditionally been evaluated based on animal models and two-dimensional (2D) cell cultures. However, animal models often fail to accurately predict human outcomes due to significant biological differences between humans and animals. For instance, while a mouse's heart beats at 600 beats per minute (bpm), a human's averages around 70 bpm. Differences in metabolism and substance tolerance can lead to inconsistent and sometimes misleading experimental results. Moreover, animals do not naturally develop many lifestyle-related diseases common in humans. To study such conditions, animals must first be artificially induced into a diseased state – for example, rats are subjected to electric shocks to mimic depression or mice undergo induced arterial blockages in the brain to replicate a stroke. Despite these manipulations, the complexity of human diseases remains inadequately represented. When experimental substances fail in later clinical stages, millions of euros in research costs are lost. At the same time, potentially life-saving drug candidates may be prematurely discarded during preclinical testing due to reliance on inappropriate models.
Research into human diseases requires human-based models
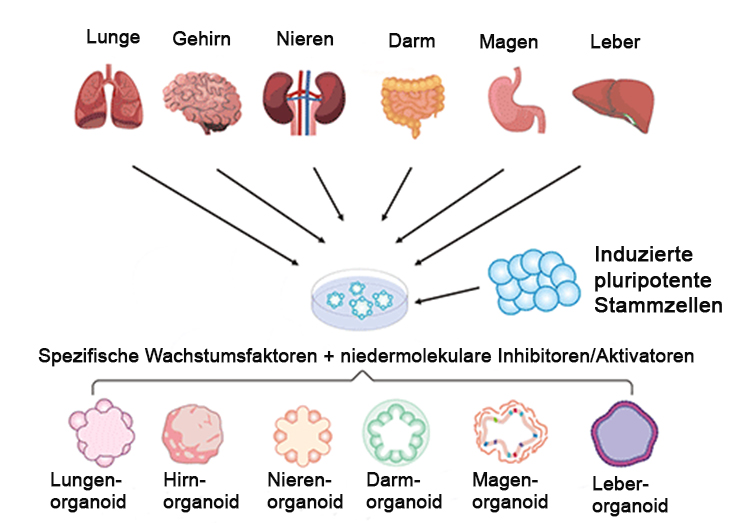 Cells are obtained from tissue samples from which pluripotent stem cells can be generated. With these cells and specific growth factors, almost all organoids can be generated. © Stephanie Heyl, modified from https://www.cusabio.com/Organoids.html
Cells are obtained from tissue samples from which pluripotent stem cells can be generated. With these cells and specific growth factors, almost all organoids can be generated. © Stephanie Heyl, modified from https://www.cusabio.com/Organoids.htmlOrganoids and organ-on-chip (OoC) systems are tiny three-dimensional, microphysiological systems that mimic the structure and behaviour of their corresponding human organs. In the case of organoids, they are three-dimensional, self-organising clusters of cells that perform specific functions and interact in a coordinated manner while retaining the physiological and genetic characteristics of the donor organ. They can replicate both healthy and specifically diseased tissue, making them invaluable for drug research, especially in the world of personalised medicine. Organ-on-chip systems, on the other hand, are microfluidic devices that replicate the functional units of organs. These systems simulate a natural cellular microenvironment where cells interact dynamically. A culture medium, acting as a substitute for blood, flows through these systems to provide essential oxygen and nutrients, closely mimicking in vivo conditions. Both technologies have an enhanced relevance to human biology. are typically more cost-effective than traditional methods and provide faster results through high-throughput screening capabilities. This efficiency enables researchers to identify the most effective therapies faster. Moreover, pharmaceutical companies like the fact that such methods use smaller quantities of test substances.
Baden-Württemberg is the first German state to establish 3R centres
The 3Rs (replace, reduce, refine) principle is a cornerstone of EU legislation aimed at improving animal welfare in scientific research. This principle seeks to replace animal experiments with alternative methods whenever possible, reduce the number of animals used to an absolute minimum, and refine procedures to ensure minimal suffering and stress for the animals involved. The Baden-Württemberg Ministry of Science, Research and the Arts (MWK), in collaboration with universities, is spearheading initiatives to implement the 3Rs on a broad scale by establishing a state-wide network. This ambitious programme began in 2020 with the launch of the 3R-Center for In Vitro Models and Animal Testing Alternatives in Tübingen. This included the creation of a bridge professorship for Organ-on-Chip research at the University of Tübingen in collaboration with the NMI Natural and Medical Sciences Institute in Reutlingen. Building on this foundation, four additional centres were established in 2021 at the Interdisciplinary Center for Gut Health (IZDG) in Heidelberg, the Center for Alternatives to Animal Testing (CAAT-Europe) in Constance, the 3R Center Rhine-Neckar, spanning Heidelberg and Mannheim, and the 3R-BioMedicUS in Stuttgart.
In addition to the 3R centres, funding had also been given to three research and two teaching projects in Freiburg, Heidelberg, Reutlingen and Ulm. Since August 2024, Dr. Silke Riegger has been head of the network office and plays a pivotal role in advancing its collaboration and strategic development. Starting in January 2025, the Karlsruhe, Furtwangen and Ulm regions will further enhance 3R competencies by establishing new centres dedicated to pursuing these principles. This expansion aims to reinforce 3R expertise in the next generation of scientists.
Animal-friendly research in Baden-Württemberg
 Since August 2024, Dr. Silke Riegger has headed up the 3R network office in Baden-Württemberg, where she is responsible for expanding networking efforts and overseeing public relations initiatives. © Dr. Silke Riegger, Werbefotografie Patrick Hipp.
Since August 2024, Dr. Silke Riegger has headed up the 3R network office in Baden-Württemberg, where she is responsible for expanding networking efforts and overseeing public relations initiatives. © Dr. Silke Riegger, Werbefotografie Patrick Hipp.The establishment of the first 3R centre in Tübingen and the bridge professorship held by Prof. Dr. Peter Loskill have significantly advanced the development and application of organ-on-chip (OoC) systems. In recognition of their ground-breaking work in this field, Prof. Loskill and Dr. Riegger received the prestigious Ursula M. Händel Animal Welfare Prize in 2024, one of Germany's most valuable research awards, worth €80,000. Among the innovations achieved by Loskill's team is a breast cancer-on-chip model for studying tumour interactions with immune cells, cytokine release and the efficacy of CAR T-cell therapies. A retina-on-chip model has also been developed to test drugs for secondary toxicity, helping to identify potential side effects early in the pro-cess. A Core Facility for Microphysiological Systems has been established at the University of Tübingen to further disseminate this cutting-edge technology through the provision of low-threshold access to researchers. Educational initiatives include a dedicated 3R module for students, the biennial conference THE 3R-LÄND and hosting the EU-ROoCS conference organised by the European Organ-on-Chips Society.
At the 3R Center Rhine-Neckar, based at Heidelberg University and the Central Institute of Mental Health (ZI) in Mannheim, the 3R principle has been expanded into a comprehensive 6R framework to further enhance animal welfare. This extension includes registration (mandatory registration prior to studies), reproducibility (repeatability of results) and reporting (publishing all results, including negative findings). Researchers at the centre are focussed on improving how mice are handled and refining methods that are used to accurately assess the burden of animal experiments. For instance, rodents can now undergo noninvasive imaging using MRI scanners, thereby minimising stress and discomfort. Dr. Marcus Meinhardt, Head of the Institute of Psychopharmacology at the ZI and coordinator of the 3R Center Rhine-Neckar, is leading efforts to evaluate new drug candidates for treating substance use disorders. The centre also serves as a hub for expertise in the design of new animal experiments, relying on in silico methods to replace and reduce animal use wherever possible.
Prof. Dr. Monilola Olayioye, spokesperson for the 3R-BioMedicUS Stuttgart, is pioneering the development of advanced 3D tumour models that replicate the complex heterogeneity of human cancers. The centre also uses high-precision bioprinting techniques that combine biomaterials with cells to engineer tissue structures, and vascularised plant vessels as innovative blood vessel models for research. Dr. Anke Burger-Kentischer at the Fraunhofer Institute for Interfacial Engineering and Biotechnology (IGB) in Stuttgart is advancing the creation of a 3D skin model, which incorporates a reporter gene built into the cells, enabling precise and direct measurement of reactions to substances. Her ground-breaking work, conducted in collaboration with Beiersdorf AG, was awarded Hamburg’s Research Prize for Alternative Methods to Animal Testing in 2024.
The 3R centre NAM-ACCEPT at CAAT-Europe in Constance focusses on developing test strategies for detecting developmental neurotoxicity. Under the leadership of Prof. Dr. Marcel Leist, the centre has pioneered an animal-free test battery based on human cells that is capable of screening nearly 200 substances through a high-throughput procedure. Additionally, in-vitro neurone models have been developed to simulate biokinetic processes such as drug distribution, with results analysed using advanced mathematical modelling. The centre’s human-based Monocyte Activation Test (MAT) has long become a reliable method for detecting fever-inducing substances in drugs. This test will fully replace the previously used rabbit test from July 2025.
 A model of a cervix has been developed on the Cervix-on-Chip. Substances are supplied and removed through the small channels. © 3R-Center Tübingen
A model of a cervix has been developed on the Cervix-on-Chip. Substances are supplied and removed through the small channels. © 3R-Center TübingenBioprinting is used at the 3R-BioMED Lab in Reutlingen to integrate liver cells into OoC chips for testing the hepatotoxic effects of drugs. In addition to this, the lab has developed a range of other OoC systems. The lab is aiming to publish project results on YouTube videos, created by and for students, to enhance knowledge-sharing and engagement. Reutlingen is also pioneering the in vitro production of sustainable fish alternatives that match the taste and nutritional value of real fish products but are toxin- and drug-free.
The Freiburg University Medical Centre has developed an innovative, non-animal safety assessment for cell-based cancer therapies. This model enables researchers to observe how CAR T-cells influence cytokine release syndrome without using animals. Another key area of work is the creation of predictive models for spinal cord injuries, where efforts are being made to systematically reduce scientific bias.
Eight Baden-Württemberg 3R centres being established
The newly established iR Centre (integrative and innovative methods to replace animal testing) in Ulm is creating a state-of-the-art refinement laboratory focussed on tumour and trauma research, where animal stress, such as cortisol levels, will be continuously monitored. The centre is also advancing organoid technologies and establishing a biobank for the exchange of animal tissue. Prof. Dr. Alexander Kleger, a pancreatic cancer specialist, is using pancreatic organoids to identify biomarkers that can detect tumours at the earliest stages. Additionally, the centre features a Core Facility for Organoids, where researchers can establish, cultivate and test organoids for various substances.
The 3ROCKIT in Karlsruhe, which focusses on computer modelling, will also open in January 2025. The idea is to create digital twins and autonomous laboratories for researching in vitro methods.
The 3R Development and Transfer Center for 3D Tissue Models in Furtwangen will focus on conducting pharmaceutical tests using artificially simulated bone substance and skin tissue. In addition to its research efforts, the centre will provide interdisciplinary teaching and advisory services for students. Prof. Dr. Margareta Müller, who will head up the centre, has specialised in in vitro models as animal alternatives for over 30 years. She says, "It is crucial that young scientists acquire expertise in 3R principles from the very start of their careers."
The integration of organoids, OoC technologies, computer simulations and epidemiological studies has the potential to deliver more meaningful and reliable results than traditional animal experiments. Effective networking and the exchange of expertise are essential to advancing these innovative methods and ensuring successful research and development outcomes.
Which animals were used for which purposes in 2022?
In Germany, mice accounted for the majority of animals used in experiments, making up 72% of the total, with fish, rats, rabbits and birds comprising a smaller portion. While the overall number of genetically modified animals has decreased, their proportion in relation to the total number of animals has increased. More than half of the animal experiments were conducted for basic research, with a smaller portion focussed on disease research and quality control and safety testing of medical products. In applied research, cancer research led the field, accounting for 42% of animal experiments, followed by studies on nervous and mental disorders (12%) and infectious diseases (10%).