Targeted, virus-free gene therapy for the eye using degradable nanopropellers
Eye diseases that result in blindness in young people are primarily caused by genetic mutations. An interdisciplinary team of researchers from the Universities of Tübingen and Heidelberg is developing an innovative gene therapy method using biodegradable, magnetic nanopropellers. These innovative nanopropellers can effectively deliver intact genes into the affected cells, offering a potential solution for treating genetic disorders of this kind.
Approximately 217 million people worldwide are visually impaired.1) In Germany, low vision is defined as impaired vision when a person has a visual acuity in the better eye of less than 30 percent, even when wearing glasses or contact lenses.2) Additionally, 36 million people have less than five percent of normal vision, making them legally blind according to the World Health Organization (WHO) definition. In Germany, these groups are not officially recorded, so the number of affected individuals can only be estimated based on WHO data or disability card applications. According to the German Association for the Blind and Visually Impaired, the estimated number of people with visual impairment in Germany is between 550,000 and 1.2 million.2)
In low-income countries, the main causes of severe visual impairment are uncorrected refractive errors, infections, cataracts, glaucoma and diabetes, often due to inadequate medical care. In contrast, in Germany, age-related macular degeneration (AMD) is the leading cause of severe vision loss, followed by glaucoma and diabetic retinopathy.1),2)
Gene therapy treatment for eye diseases

"Retinal diseases that lead to blindness at a young age are typically genetic," explains Dr. Sven Schnichels from the Department of Ophthalmology at the University Hospital of Tübingen. "These conditions usually manifest in childhood or adolescence and are characterised by the rapid degeneration of photoreceptors or retinal pigment epithelial cells. Over 2 million people worldwide are affected by monogenic retinal diseases, where a single gene is mutated. In such cases, therapies that introduce a functional gene into the retinal cells can be highly effective."
The first gene therapy drug in ophthalmology was approved in 2017.3) It is used to treat Leber congenital amaurosis (LCA), a specific form of retinal dystrophy within the broader group of retinitis pigmentosa (RP). Retinal dystrophies are caused by structural defects in various proteins essential for the function of the visual pigment rhodopsin. As a result, the rod cells, which specialise in light perception, and later the cone cells, responsible for colour vision, progressively degenerate, leading to gradual vision loss.
Current therapy for Leber congenital amaurosis (LCA) employs genetically modified adeno-associated viruses (AAV) as a delivery vehicle to introduce a healthy version of the mutated RPE65 gene into retinal cells. In the laboratory, most of the viral genome is replaced with the human gene. After the AAV is injected into the eye, the virus enters retinal cells through its outer protein structure. The DNA that has been delivered into the cells serves as a template for producing a functional protein. This approach has led to significant improvements in rod cell function in what was previously an incurable disease. However, the long-term effect remains unclear.
Virus-free nanopropellers as transport vehicles
"However, it has since become clear that the treatment is not as efficient as expected, and that AAVs, although harmless to humans, can sometimes trigger immune reactions," says the biologist. "Moreover, the procedure is not suitable for larger genes. Since the injections are administered subretinally, meaning beneath the retina, the vitreous body must be removed beforehand." As a result, he and his colleague Dr. José Hurst, along with the research group led by physicist Prof. Dr. Peer Fischer from the Max Planck Institute for Medical Research in Heidelberg, began developing an alternative approach in 2018.
The researchers' virus-free gene therapy approach is based on nanopropellers - tiny, spiral-shaped particles in the size range of bacteria that can be propelled and controlled thanks to their magnetic properties. In the REVeyeVE project, funded by the German Federal Ministry of Education and Research under the GO-Bio initial programme, the team first assessed the economic aspects of the project before starting to develop the proof of concept.
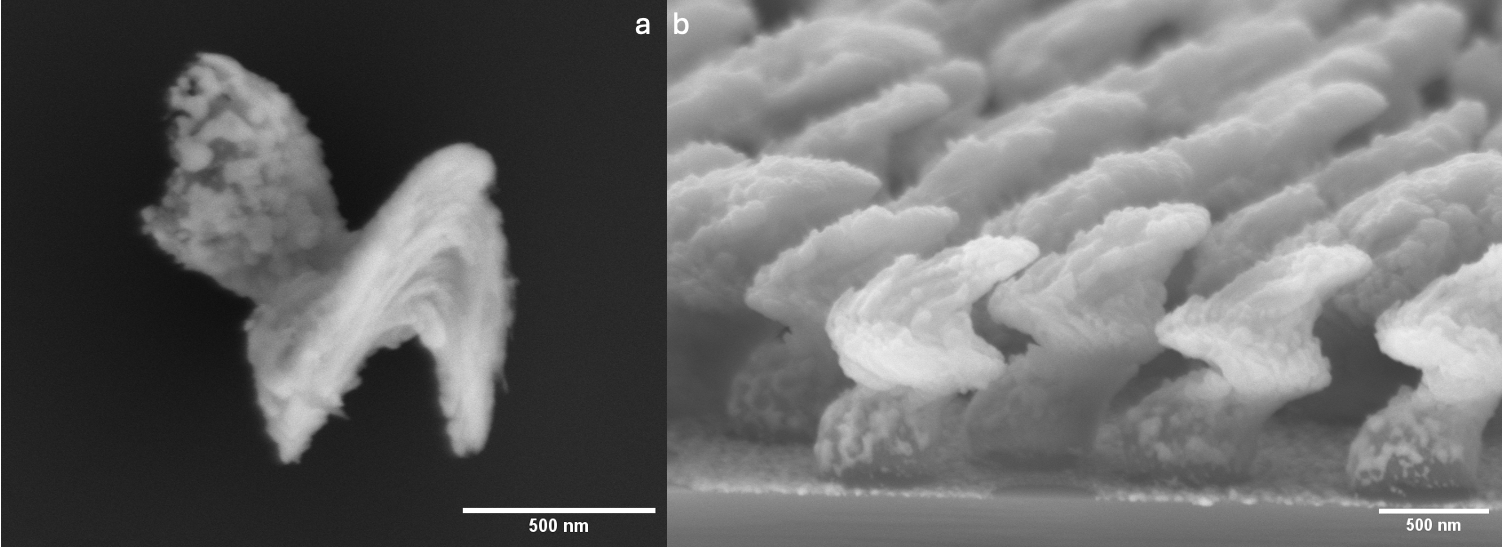
In the initial phase of their research, the team developed a method for producing the particles from biodegradable materials. "Our nanopropellers measure around 1200 nm in size, and are made from magnesium and zinc. Apart from the iron-containing core, the nanopropellers can be broken down by the body; some components can even be utilised," explains Florian Peter, a doctoral student in Heidelberg. "Depending on the ratio of magnesium to zinc, the particles can have a lifespan ranging from 24 hours to one month, allowing them to be tailored to the specific therapy." Outside the body, the particles remain stable at room temperature for several months.4)
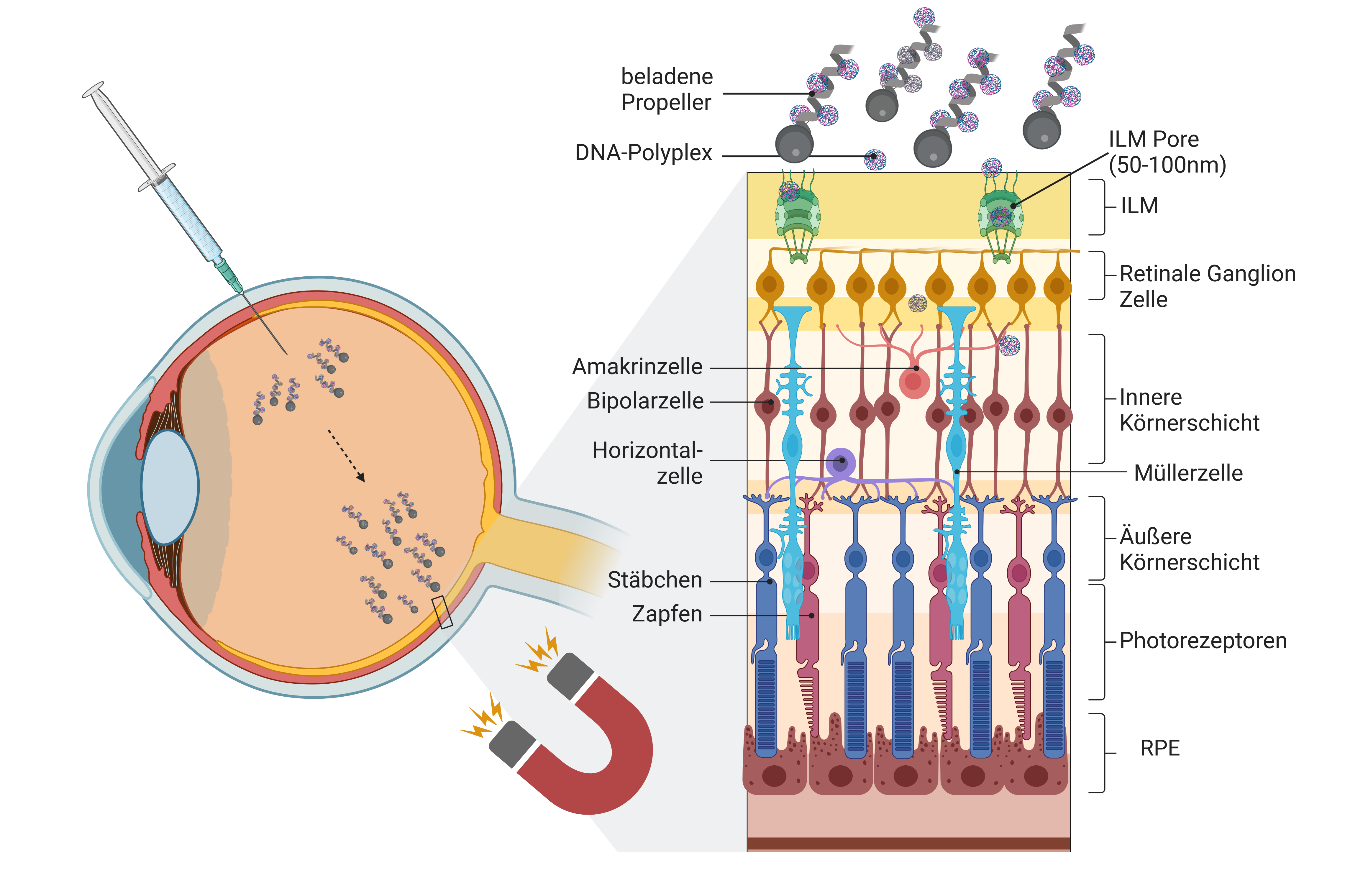
The excellent biological compatibility of the nanopropellers has been confirmed in laboratory tests, and they have now been successfully loaded with DNA. "Since not only the gene itself but also regulatory sequences must be delivered into the cells, the plasmids - ring-shaped nucleic acid molecules - can be up to 1500 nm in diameter, making them larger than the transport vehicle," explains Hurst. The researchers use synthetic polymers that form polyplexes with the DNA to reduce the size of the plasmids. Tightly packed inside the polyplexes, the genetic material now has a size of 20-50 nm. It is expected that individual nanopropellers can bind and transport up to ten of these polyplexes. The cargo is released during the degradation process.
Hurst emphasises: "We are currently working with a plasmid that encodes the rhodopsin gene. However, in principle, our technology can be used to package any type of nucleic acid, including various RNA variants. We are not focused on creating a specific therapeutic agent; instead, our efforts are concentrated on establishing the platform, optimising the packaging unit, and ensuring its effective binding to the nanoparticles."
Innovative drug delivery system with great potential
In July 2024, these promising results earned the REVeyeVE project second place in the annual Science2Start ideas competition, organised by BioRegio STERN.5) In addition, project funding was extended by another six months, taking it to late March 2025. "We are now beginning preclinical applications and still need to fine-tune the magnetic control system and loading mechanisms," explains Schnichels. Further experiments will initially be conducted on isolated pig eyes, which closely resemble human eyes in both structure and size. After injection into the vitreous body, the nanopropellers will be directed to the target cells using magnetic fields. "We use small fields that can be controlled by magnetic coils," says Lucie Motyćkova, a doctoral student in Heidelberg.
The team is optimistic that the process will be refined enough to enable clinical studies within a few years. Since the nanopropellers can transport a wide variety of nucleic acids in a targeted manner without significant size limitations, their potential applications extend well beyond ophthalmology, opening up possibilities for use in other medical fields.
References:
1) VISION 2020 brochure (as of 2019). https://www.woche-des-sehens.de/fileadmin/Redaktion/Materialien/2019/Broschuere_VISION2020_09_2019_barrierefrei.pdf
2) German Association for the Blind and Visually Impaired: Zahlen & Fakten. https://www.dbsv.org/zahlen-fakten.html
3) Augenärztliche Akademie (AAD): Press conference 2020. https://www.augeninfo.de/offen/pressetext_aad.php?meldung=260
4) Peter, F. et al. (2024): Degradable and Biocompatible Magnesium Zinc Structures for Nanomedicine: Magnetically Actuated Liposome Microcarriers with Tunable Release. Adv. Funct. Mater. 34, 2314265. https://doi.org/10.1002/adfm.202314265
5) BioRegio STERN: Science2Start. https://www.bioregio-stern.de/de/projekte/science2start