Prostate cancer is the most commonly diagnosed cancer in men, accounting for 26% of cases. Once metastasised, it is considered incurable. Cancer immunotherapy offers a promising approach, utilising the body's own resources to target tumour structures while sparing healthy tissue. Research into the efficacy and safety of immunotherapies for solid tumours is ongoing. The aim is to replace standard therapies, which often come with side effects. When it comes to localising the tumour, antibodies are essential as they identify unequivocally the tumour's surface proteins (antigens). Bispecific antibodies are laboratory-created constructs that do not occur naturally in living organisms. Their unique structure allows them to recognise two distinct target antigens and, for example, facilitate the binding of immune cells, such as T cells, to the tumour.
Antibody development by TWYCE and BiconY
 Their goal is to improve cancer immunotherapy: Prof. Dr. Helmut Salih, Prof. Dr. Gundram Jung and Dr. Martin Pflügler (from left to right) founded the company TWYCE GmbH and are developing new bispecific antibodies at the University of Tübingen’s Medical Institute and the German Cancer Research Center (DKFZ) in Heidelberg. © Mattia Balsamini
Their goal is to improve cancer immunotherapy: Prof. Dr. Helmut Salih, Prof. Dr. Gundram Jung and Dr. Martin Pflügler (from left to right) founded the company TWYCE GmbH and are developing new bispecific antibodies at the University of Tübingen’s Medical Institute and the German Cancer Research Center (DKFZ) in Heidelberg. © Mattia BalsaminiTWYCE is supported by the Federal Agency for Leap Innovations (SPRIN-D), which funds applied research projects aimed at providing innovative solutions to societal challenges. A company called BiconY was specifically established as an intermediary between TWYCE and SPRIN-D to facilitate this funding, as federal institutions are prohibited from directly investing in private companies. Funding from the federal budget is allocated to BiconY, with research objectives defined by TWYCE under a cooperative agreement between the two entities. TWYCE is recognised as a trailblazer in cancer immunotherapy for solid tumours, and is pioneering a novel approach involving two synergistic bispecific antibodies. This combinatorial strategy aims to enhance the effectiveness of T-cell-based immunotherapy while mitigating potential side effects.
The three founders of TWYCE are distinguished researchers from the Clinical Cooperation Unit (CCU) Translational Immunology at the University of Tübingen and the German Cancer Research Center (DKFZ): Prof. Dr. Gundram Jung, Prof. Dr. Helmut Salih and Dr. Martin Pflügler. Professors Jung and Salih specialise in the advancement and clinical translation of therapeutics, focusing on immunology. Dr. Pflügler, leveraging his expertise as a pharmacist, contributes extensive knowledge in antibody production and regulatory compliance.
Monotherapy with one antibody is often not sufficient
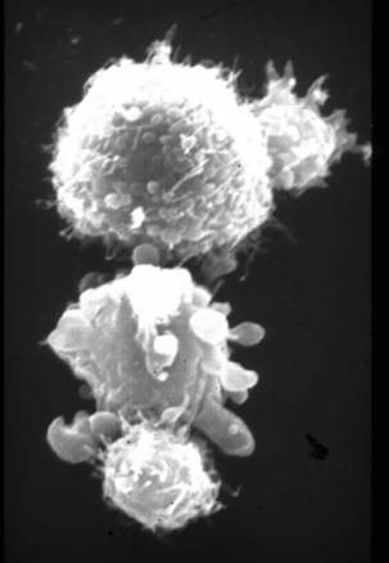 The two smaller T cells (top and bottom right in the picture) attack the two larger tumour cells, whereby the lower cancer cell is already undergoing lysis. © Prof. Dr. Gundram Jung, TWYCE GmbH
The two smaller T cells (top and bottom right in the picture) attack the two larger tumour cells, whereby the lower cancer cell is already undergoing lysis. © Prof. Dr. Gundram Jung, TWYCE GmbHThe three researchers have devised an innovative therapy concept aimed at swift clinical implementation. While they have achieved notable success in various types of blood cancer, tackling solid tumours poses distinct challenges. These tumours are generally hard for immune system effector cells to reach outside the vascular system, and they lack specific antigens exclusive to the tumour cells. "The tumour comprises the body's own cells and proteins; it doesn't generate own antigens," explains Pflügler.
Another challenge lies in sustaining T-cell activation within the immune response against cancer. In a recent study involving patients with advanced prostate cancer, a bispecific antibody was administered. This antibody binds both the prostate-specific membrane antigen (PSMA) and the CD3 receptor on T cells, facilitating close interaction between these cells to trigger a response. Elevated levels of prostate-specific antigen (PSA) serve as a reliable biomarker for prostate cancer, providing early indications of recurrence even after prostate removal. "While the therapy initially shows promise - activating T cells to attack the tumour - this activation is short-lived," explains Pflügler. "After activation, the immune system is carefully regulated to prevent overactivation, which could lead to cytokine release syndrome (CRS), a potentially life-threatening condition." Consequently, T cells become inactive shortly after the initial response, halting their attack on the tumour.
For patients with advanced carcinoma, the initial approach was insufficient to combat the tumour effectively. Jung, Salih and Pflügler are currently conducting a follow-up study to investigate whether monotherapy with a bispecific antibody could control cancer cells in patients with a lower tumour burden. "These patients are experiencing biochemical recurrence," explains Pflügler. "Although the prostate has been removed, elevated PSA levels indicate the presence of residual tumour cells." The researchers are optimistic that in such cases, a bispecific antibody could be sufficient to manage the disease.
Improved efficiency through dual stimulation
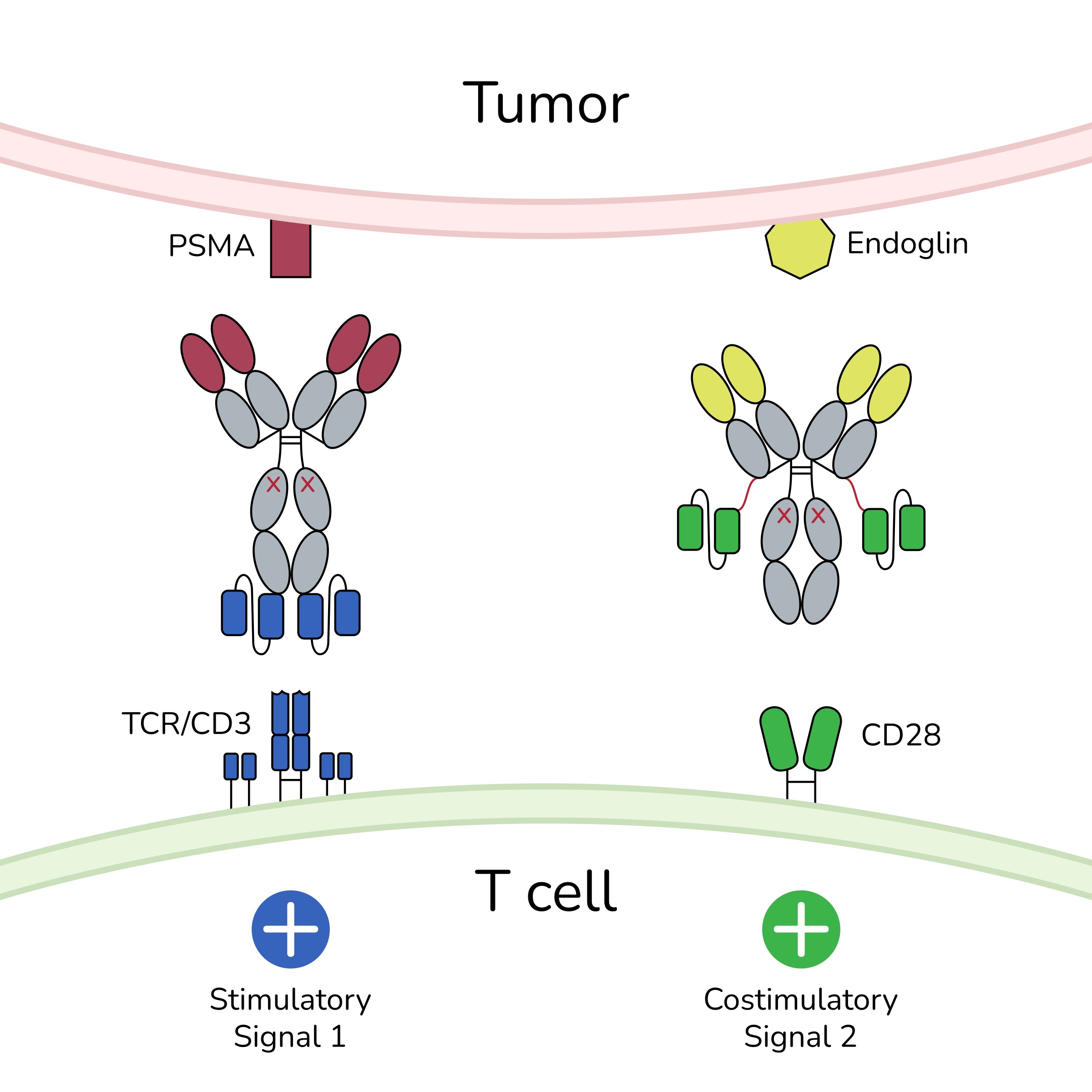 The bispecific antibody, in conjunction with a bispecific costimulator, establishes spatial proximity between T cells and tumour cells by binding to their respective receptors (CD3 and CD28 on T cells, and PSMA and endoglin on tumour cells). This interaction generates signals that trigger a reaction, leading to the lysis of the tumour cell. © TWYCE GmbH
The bispecific antibody, in conjunction with a bispecific costimulator, establishes spatial proximity between T cells and tumour cells by binding to their respective receptors (CD3 and CD28 on T cells, and PSMA and endoglin on tumour cells). This interaction generates signals that trigger a reaction, leading to the lysis of the tumour cell. © TWYCE GmbHPflügler has already started the next trial with TWYCE for patients with a higher tumour burden. Depending on whether the many regulatory requirements are met, this trial is scheduled to start in late 2024 or early 2025 and will show how the aforementioned monotherapy weaknesses can be overcome. The idea is to use a second bispecific antibody as a costimulator that recognises a second target on the tumour and T cell and also maintains the response over a longer period of time. Accessibility is increased by addressing the target antigens not only on tumours but also on tumour vessels.
"This approach facilitates tumour access, as the immune cells open the vessels specifically at the tumour site," explains Pflügler. To prevent the immune reaction from subsiding, the second antibody generates an additional signal via the CD28 receptor on the T cell, alongside the CD3 signal. "This costimulatory signal prevents the T cell from shutting down, thereby sustaining a longer-lasting immune response," Pflügler adds.
The surface antigens PSMA and endoglin, found together exclusively in prostate carcinomas, are targeted by the two bispecific antibody constructs. These antibodies bring T cells into close proximity with the tumour tissue. The costimulator is only activated if the initial signal via CD3 antibodies is present. This signalling cascade induces the lysis of malignant cells, resulting in a potent, dual anti-tumour effect while minimising toxicity to healthy cells. An additional benefit is that the dosage of the primary antibody can be significantly reduced due to the costimulation, which makes the therapy safer and more efficient. As with all immunotherapies, the primary side effect here is cytokine release syndrome (CRS). However, in this case, CRS is typically mild, temporary and primarily localised to the tumour site. "The higher the tumour load, the stronger the cytokine release, as more T cells are activated," explains Pflügler. "In this context, the side effect is essentially part of the therapeutic effect." If the trials proceed successfully, this therapy could become part of routine clinical practice within around six years. It also has the potential to be combined with other antibody or peptide-based therapies, which would enhance its overall effectiveness.