SpecPlate: an innovative multiwell plate
Focus on innovative drug development - accurate, time- and cost-saving
Drug research is a complex and costly process that requires extensive laboratory analyses and vast quantities of consumables, with disposable sample carriers in particular being used in their millions. Karlsruhe-based PHABIOC GmbH has come up with a solution in the form of the SpecPlate, a ground-breaking alternative to sample carriers that significantly reduces the time taken to perform analyses as well as the quantity of materials consumed. Serial production is already underway.
Biopharmaceuticals – drugs developed and produced using biotechnological methods – are a rapidly expanding market.¹) They are already widely used across most medical fields, particularly as antibody therapies and vaccines. However, developing them is highly time-consuming and resource-intensive. Optimising this process would be beneficial not only for the pharmaceutical industry but also for patients, who stand to gain from these next-generation therapies that target biological processes more precisely than conventional drugs.
A key step in developing such substances involves measuring the concentration and purity of dissolved samples. The current laboratory standard for this process is UV spectroscopy, typically performed in 96-well microtitre plates for high-throughput analysis. These sample carriers, made from high-quality plastics, are discarded after use, resulting in significant resource consumption. Moreover, the method is not particularly accurate: variations in pipetting can lead to inconsistent filling of the measuring chambers, and liquid menisci – curved surface tensions – can cause measurement errors. Additionally, samples are often too concentrated, requiring dilution steps that introduce further inaccuracies and inefficiencies.
Innovative solution aims to become a better standard
Many years ago, bioengineer Dr. Carsten Radtke identified a need for a more practical alternative in everyday laboratory work. During his doctorate at the Karlsruhe Institute of Technology (KIT), he began using 3D bioprinting, automation and microfluidics to develop innovative sample carriers. These carriers address the limitations of the current gold standard while remaining fully compatible with conventional pipetting robots and standard measuring devices – thus eliminating the need for costly new equipment.
 Jannik Jungmann (left) and Dr. Carsten Radtke (right) co-founded PHABIOC, a start-up company specialising in technology transfer that has already successfully brought innovative products to market. © PHABIOC GmbH
Jannik Jungmann (left) and Dr. Carsten Radtke (right) co-founded PHABIOC, a start-up company specialising in technology transfer that has already successfully brought innovative products to market. © PHABIOC GmbHThe bioengineer’s efforts have paid off: Radtke’s research has not only led to a concrete product – the SpecPlate, which recently entered serial production – but also to the founding of PHABIOC GmbH in Karlsruhe. Established in 2023 as a spin-off from KIT, the company was launched in collaboration with biomedical engineer Jannik Jungmann. "The breakthrough moment came in 2017 when Carsten presented the SpecPlate at a high-throughput conference and received overwhelmingly positive feedback from experts. That’s when we decided to turn the concept into a market-ready product. With support from a publicly funded ZIM project, we initiated development work," Jungmann recalls. "By 2021, we had created the first functional prototypes and gathered valuable feedback from field test customers. However, transitioning to full-scale production proved challenging. We had to establish an injection moulding process under cleanroom conditions for extremely small chamber heights, which was no mean feat," explains Jungmann. "After extensive quality assurance testing, we have now officially launched serial production. The SpecPlate is available to everyone and is manufactured in Germany."
Plug and play as well as fast, precise and cost-saving
 At first glance, the SpecPlate resembles a conventional microtitre plate. However, unlike standard designs, each of its 96 measuring structures features a step-like channel system, enabling significantly more efficient measurements. © PHABIOC GmbH
At first glance, the SpecPlate resembles a conventional microtitre plate. However, unlike standard designs, each of its 96 measuring structures features a step-like channel system, enabling significantly more efficient measurements. © PHABIOC GmbHWhat sets the SpecPlate apart from conventional microtitre plates? Like many standard UV plates, it is made of COC (cyclic olefin copolymer). However, unlike traditional designs, each of its 96 measuring chambers is a minimally small, closed, step-like channel system. This innovative structure allows precise filling with just 36 µl of sample solution, eliminating measurement errors caused by inaccurate pipetting or liquid menisci. While conventional cuvettes offer similar precision, they cannot be automated – giving the SpecPlate a distinct advantage in high-throughput applications.
In addition, each of the 96 structures features four different chamber heights, enabling four independent concentration measurements per sample. This results in a total of 384 measuring points, allowing for a broad concentration range without the need for error-prone sample dilution. "These details save an enormous amount of time and materials – 75% less laboratory consumables, a 30% reduction in process time and improved data quality," explains Jungmann. "Moreover, the SpecPlate can be integrated into all laboratory devices, including pipetting machines and plate readers, almost like plug and play. These advantages should more than offset the additional cost when compared to conventional plates."
The experts behind the SpecPlate, whose promotional slogan is "We are the better standard," are actively seeking additional pilot customers to showcase the advantages of their innovative sample carrier. "We already have customers, but new ones are very welcome," says co-founder Jungmann. "User feedback is crucial for us." Now that the microtitre plate is available as a serial product, the next step is to collaborate with customers to develop tailored solutions for new applications. In recognition of their ground-breaking innovation, the PHABIOC team was awarded the 2024 Baden-Württemberg Founder Award.
Biomimetic barriers complete efficient laboratory analysis
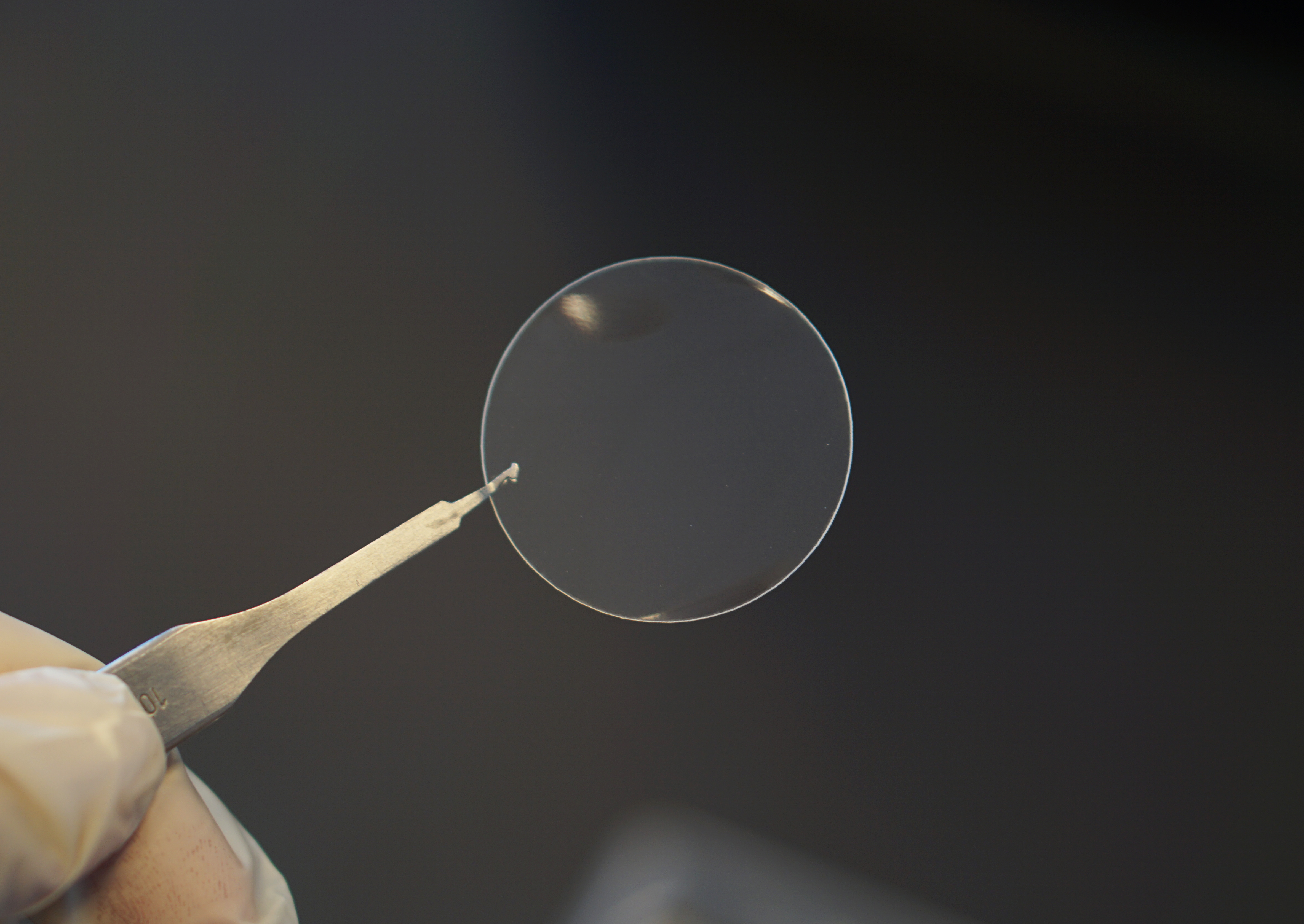 The PermeaPad® GIT is an artificial biomimetic barrier that simulates the conditions at the interfaces of the gastrointestinal tract and thus enables investigations into the body’s absorption of active ingredients in this area. © PHABIOC GmbH
The PermeaPad® GIT is an artificial biomimetic barrier that simulates the conditions at the interfaces of the gastrointestinal tract and thus enables investigations into the body’s absorption of active ingredients in this area. © PHABIOC GmbHThe SpecPlate is one of several innovations in PHABIOC’s portfolio. The young company – whose name stands for PHArma, BIOtechnology, Consumables – has made technology transfer its specialty, as Jungmann explains. Another of PHABIOC’s ground-breaking technologies – the PermeaPad® – came from the biotechnology company innoMe, which initially served as production partner for the SpecPlate and was where the two founders first met. These in vitro permeation assays are fully synthetic biomimetic membranes that replicate different biological barriers, such as mucous membranes in the gastrointestinal tract (GIT), nose, skin and mouth, precisely simulating how active substances are absorbed into the body. They are designed not only to accelerate drug development but also to advance the 3Rs principle (Replacement, Reduction, Refinement) in animal testing.
The GIT variant has been on the market since 2019, in the form of pads for standard diffusion cells or in 96-multiwell plates for high-throughput screening of drugs. The development of the skin model, which was launched in 2023, is due to be completed shortly.
"Users in both academic and industrial research have already given positive feedback and published numerous studies," says Jungmann. "Moving forward, we aim to collaborate with our customers to develop and implement even more advanced barriers."