New treatment methods: DNA origami-based nanodevices precisely control immune response
Bottom-up synthetic immunology for novel therapeutic approaches
Modern therapies for combating cancer and infectious diseases increasingly leverage the body’s own immune system. Several research groups at Heidelberg University are using innovative bottom-up approaches in synthetic immunology to develop new treatment methods that can control the immune response more precisely than previously possible.
While our immune system plays a crucial role in recognising and combating pathogens or cancer cells, it sometimes requires additional support. Vaccines, for example, help prime the immune system by sensitising it to specific pathogens, enabling it to quickly recognise and eliminate the pathogens when exposed to them. However, cancer cells are more challenging for the immune system to detect, as they often resemble healthy cells with only minor differences. Moreover, tumour cells frequently possess mechanisms that can evade detection or block the immune response, making them harder to eliminate.
Modern cancer therapies increasingly focus on overcoming the immune evasion mechanisms employed by tumour cells. Immuno-oncology leverages several innovative strategies: checkpoint inhibitors block inhibitory signalling molecules on cancer cell surfaces, restoring the immune system’s ability to recognise and attack them. Bispecific antibodies create a direct link between immune and tumour cells, enhancing immune activation through spatial proximity. CAR T-cell therapy takes a more personalised approach, genetically modifying a patient’s own T cells to specifically recognise and destroy cancer cells. While these methods have shown remarkable success, they can also cause significant side effects.
Supporting the immune system with the help of DNA origami
 Prof. Dr. Kerstin Göpfrich and her research group at the ZMBH are creating novel molecular building blocks to assemble cellular structures and, in the long term, a complete artificial cell from scratch. © Kathrin Hall, ZMBH
Prof. Dr. Kerstin Göpfrich and her research group at the ZMBH are creating novel molecular building blocks to assemble cellular structures and, in the long term, a complete artificial cell from scratch. © Kathrin Hall, ZMBH"Modern, cell-based cancer therapies like CAR T-cell therapy follow a top-down strategy. While highly effective, these treatments are extremely costly and their effects cannot always be precisely controlled," explains Prof. Dr. Kerstin Göpfrich from the Center for Molecular Biology at Heidelberg University (ZMBH). "That's why we are taking a bottom-up approach - using simple molecular building blocks to construct cellular structures, with the long-term goal of creating a fully synthetic cell from scratch." With her Biophysical Engineering of Life research group, the physicist has already established key foundations for this innovative approach.
The innovative nanodevices developed by the researchers are primarily based on DNA origami. This technique uses long single strands of DNA - typically of viral origin - which are folded into intricate three-dimensional structures with the help of short synthetic DNA strands. These structures can perform specific functions, including those typically carried out by proteins in natural cells.1)
Göpfrich explains: "DNA origami allows us to create molecular hardware relatively easily and cost-effectively. Since we work exclusively with well-defined components, we maintain full control over the system, enabling us to precisely activate or deactivate functions as needed. In contrast, the top-down approach results in synthetic cells carrying a significant amount of unpredictable evolutionary baggage."
In 2022, the ‘Synthetic Immunology’ spotlight project was launched in order to apply the promising bottom-up engineering approaches of synthetic biology to immunology. This interdisciplinary collaboration brings together biology, physics, medicine, chemistry and pharmacy experts from Heidelberg research institutes. Utilising cutting-edge technologies, the project aims to deepen our understanding of the immune system and explore new strategies for targeted immune modulation.
Novel vaccines through a bottom-up approach
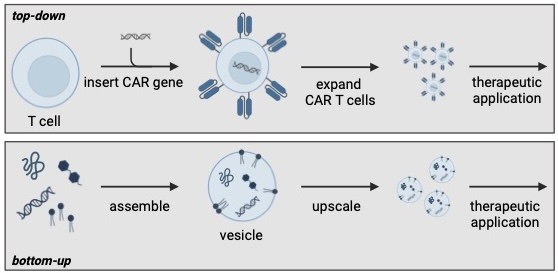 CAR T-cell therapy uses the body's own immune cells, which are genetically modified in the laboratory so that they can recognise and destroy cancer cells (top-down approach). In the bottom-up approach, therapeutically effective, cell-like vesicles are constructed from scratch using artificial building blocks. © Oliver Fackler, Michael Platten und Kerstin Göpfrich
CAR T-cell therapy uses the body's own immune cells, which are genetically modified in the laboratory so that they can recognise and destroy cancer cells (top-down approach). In the bottom-up approach, therapeutically effective, cell-like vesicles are constructed from scratch using artificial building blocks. © Oliver Fackler, Michael Platten und Kerstin GöpfrichIn a 2024 article published in Nature Nanotechnology, Göpfrich and her colleagues highlight the immense potential of this emerging field.2) One notable example of early success is the development of coronavirus SARS-CoV-2 vaccines, which use artificially produced mRNA molecules encapsulated in lipid nanoparticles. Now, researchers in Heidelberg are working on vaccines based on DNA origami. "We are designing a DNA scaffold that allows us to precisely position various antigens [structures that trigger an immune response], mimicking their natural arrangement on pathogen surfaces," Göpfrich explains. "Current vaccines typically contain only single soluble antigens, which is often insufficient for diseases such as malaria or HIV." The team is optimistic that bottom-up nanotechnology can overcome these limitations and enhance immune protection.
Peptide boronic acids open up a wide range of possibilities
 Prof. Dr. Christian Klein, Junior Prof. Dr. Franziska Thomas and doctoral student Marius Werner developed a quick and simple method for producing peptide boronic acids at Heidelberg University. © Julian Brinkhofer
Prof. Dr. Christian Klein, Junior Prof. Dr. Franziska Thomas and doctoral student Marius Werner developed a quick and simple method for producing peptide boronic acids at Heidelberg University. © Julian BrinkhoferProtein and peptide engineering is another key focus of synthetic immunology. As part of the Synthetic Immunology spotlight project, research groups led by Junior Professor Dr. Franziska Thomas at the Institute of Organic Chemistry and Prof. Dr. Christian Klein at the Institute of Pharmacy and Molecular Biotechnology at Heidelberg University are actively exploring this field. Short peptides derived from pathogen- or tumour-specific proteins play a crucial role in identifying infected or malignant cells. These peptides are displayed on the cell surface, bound to specialised proteins known as MHC molecules (Major Histocompatibility Complex), where they trigger a cytotoxic immune response. However, this response is often too weak to be effective. To overcome this limitation, the researchers are developing synthetic peptides designed to enhance and strengthen the immune reaction.
During their research, the teams developed a novel method for synthesising peptide boronic acids - peptides that incorporate a boronic acid group (-B(OH)₂). "Peptide boronic acids can form reversible covalent bonds, making them highly attractive for pharmaceutical applications," explains Thomas. "In contrast to unmodified peptides, they initially establish very stable interactions but over time these bonds hydrolyse, meaning they gradually break down in an aqueous environment." This unique property unlocks a range of potential applications. "By coupling peptide boronic acids with carrier molecules such as dextran nanoparticles, we can ensure targeted delivery and controlled release at the site of action, creating a depot effect," says Klein. "Additionally, we can fine-tune peptide-protein interactions to develop more effective inhibitors or enhance the binding of immunogenic peptides to MHC molecules, thereby improving immune response modulation."
The power of solid-phase synthesis
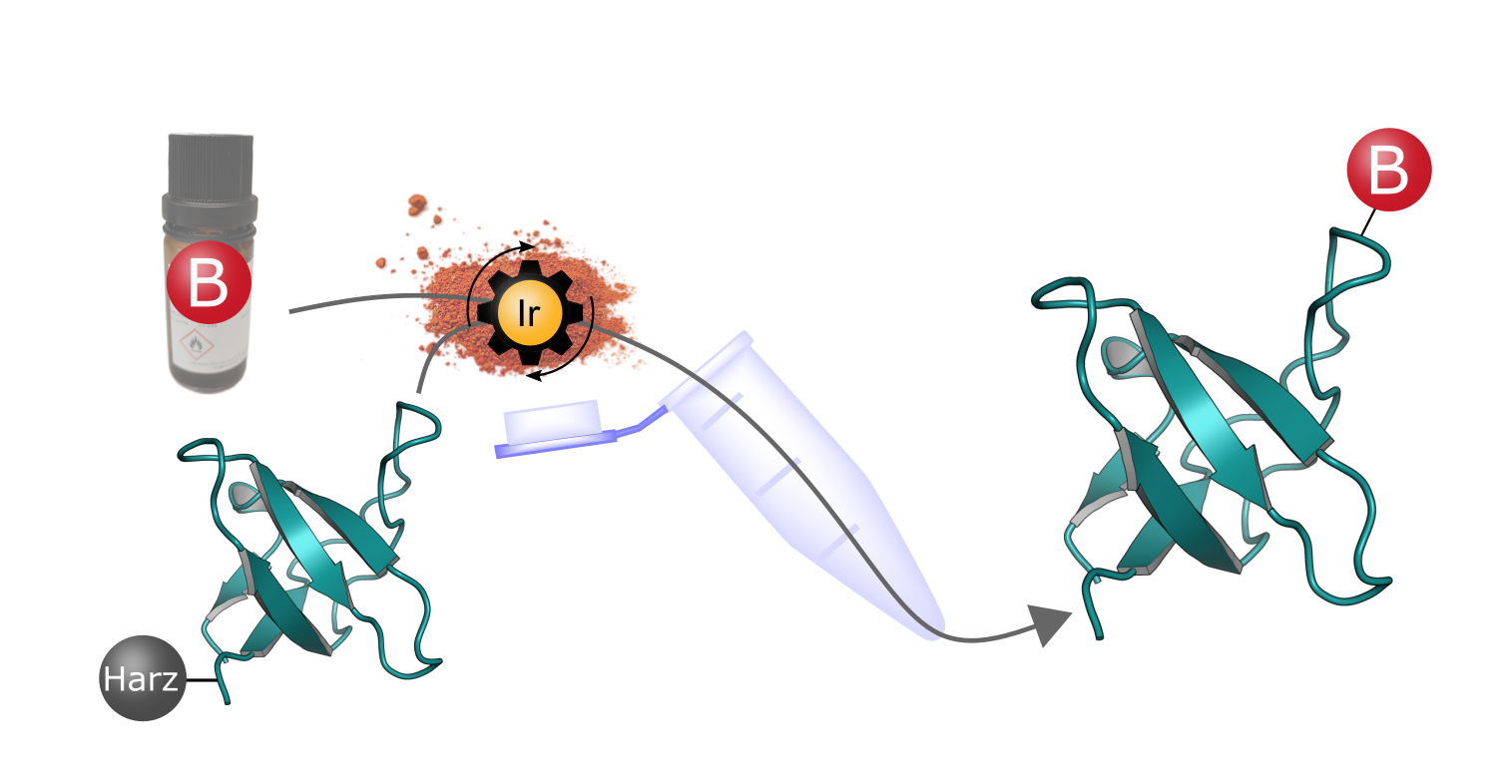 The innovative synthesis process is based on resin-bound peptides to whose reactive side chains boronic acid groups (B) are attached with the aid of an iridium catalyst (Ir). The resulting peptide boronic acids have special properties that could open up new pharmaceutical applications. © Marius Werner (based on the protein structure PDB 1CKB)
The innovative synthesis process is based on resin-bound peptides to whose reactive side chains boronic acid groups (B) are attached with the aid of an iridium catalyst (Ir). The resulting peptide boronic acids have special properties that could open up new pharmaceutical applications. © Marius Werner (based on the protein structure PDB 1CKB)Synthesising peptide boronic acids used to be extremely complicated and time-consuming. The process developed by PhD student Marius Werner, which was also published in the journal Advanced Science in 2024, is not only significantly faster, but also enables the formation of more complex molecules.3) Based on classic solid-phase peptide synthesis, the desired amino acid chain is bound to a solid resin in the first step. The boronic acid group is then added by hydroboration of reactive double or triple bonds of the side chains. As Thomas emphasises: "The process is very efficient and suitable for both short and long peptides."
Boronic acid is a very diverse functional group and can be further modified or even completely replaced, resulting in a wide range of possible applications. The researchers are planning to use these tools to work on immunological issues in particular.
Due to the close proximity between basic research and clinical application in Heidelberg, the conditions are ideal for interdisciplinary cooperation, which hugely benefits all the synthetic immunology projects.