Chronic inflammatory bowel diseases
Proinflammatory regulatory T lymphocytes as a therapeutic target in Crohn's disease
Chronic inflammatory bowel diseases are very stressful for those affected and increase the risk of bowel cancer. PD Dr. Robyn Laura Kosinsky from the Bosch Health Campus in Stuttgart, together with researchers from the USA, identified disfunctional regulatory T cells as important drivers of inflammation in Crohn's disease. They also found that with the help of an epigenetically active drug, it was possible to restore the cells’ original function.
Recurrent abdominal pain and persistent diarrhoea, often in combination with fever, loss of appetite, nausea and vomiting can all be signs of chronic inflammatory bowel disease (IBD). Six to eight million people (including two million in Europe) worldwide are estimated to be living with IBD.1) Crohn's disease (MC) and ulcerative colitis (UC) are the main types of IBD, affecting nine out of ten people. Despite causing very similar symptoms, the two diseases differ fundamentally in how they manifest: UC spreads evenly from the rectum to part or all of the colon and only affects the intestinal mucosa whereas Crohn's disease occurs in patches predominantly at the junction between the small and large intestine and can spread through all layers of the intestinal wall. However, in both forms of the disease the intestinal barrier function is compromised and a disordered immune reponse plays a crucial role in pathogenesis.
The exact pathogenesis of IBDs is not yet fully understood, but it is clear that several factors need to come together.1) In addition to a genetic predisposition, nicotine consumption (in MC) or antibiotic use in childhood and, in particular, the change in the microbiome balance (dysbiosis) in the gut associated with a Western diet appear to play a role. In the long term, chronic inflammation can impair intestine function to such an extent that the absorption of minerals such as iron and calcium or fat-soluble vitamins is reduced. Those affected also have an increased risk of bowel cancer and often suffer from additional symptoms outside the digestive tract, for example in the joints, skin, eyes or liver.
T lymphocytes regulate the immune response
 PD Dr. Robyn Laura Kosinsky (standing at the back) and her research group at the Robert Bosch Centre for Tumour Diseases in Stuttgart are investigating the molecular basis of chronic inflammatory bowel diseases. © Bosch Health Campus, Akshay Kanakan
PD Dr. Robyn Laura Kosinsky (standing at the back) and her research group at the Robert Bosch Centre for Tumour Diseases in Stuttgart are investigating the molecular basis of chronic inflammatory bowel diseases. © Bosch Health Campus, Akshay Kanakan"I want to better understand the role of the immune system in Crohn's disease," says PD Dr. Robyn Laura Kosinsky, who has been group leader at the Robert Bosch Centre for Tumour Diseases in Stuttgart since 2022. To this end, she spent two years in the USA at the Mayo Clinic in Rochester, Minnesota, funded by a Mildred Scheel Fellowship from German Cancer Aid. There she focussed on T lymphocytes, which together with the antibody-producing B lymphocytes and natural killer cells, form a subgroup of white blood cells.
T lymphocytes can be divided into T killer cells and T helper cells. T killer cells are directly involved in recognising and destroying infected or altered body cells and are characterised by the surface protein CD8. T helper cells express CD4 molecules and have no cytotoxic activity; they coordinate the acquired immune response by releasing special messenger substances known as cytokines. These stimulate other immune cells such as macrophages, B lymphocytes and cytotoxic T cells and are essential for an effective immune response.
Another group (< 10 percent) is made up of CD4-positive (CD4+) regulatory T cells (Tregs), which secrete inhibitory cytokines and curb the activity of the immune system. This is both important in order to switch the immune response off again after some time and necessary for developing self-tolerance. In chronic inflammatory diseases, the balance between activation and suppression is disturbed, causing the immune system to overshoot the mark, which is why immunosuppressants are primarily used for treating chronic inflammatory diseases.
Proinflammatory Tregs discovered
Kosinsky and her fellow researchers adopted a special approach to determine the role of CD4+ T lymphocytes in Crohn's disease. The molecular biologist explains: "As tissue samples contain a large number of different cell types, we first enriched all CD4+ cells. With the help of single-cell RNA sequencing, we were then able to determine the genes transcribed in each cell and carry out detailed analyses." They found that the proportion of Tregs in the samples of Crohn’s disease patients is significantly higher than in healthy individuals. "This is not unusual, nor should it be, as it is the Tregs' job to fight inflammation. Why they fail to do this is not yet fully understood," she says.
The scientists therefore took a closer look at what happens inside the cells. Based on the expression profile, i.e. the totality of the gene products present in the cell, they found that the Tregs of healthy and diseased people differ considerably. In total, they were able to define five different subgroups. The majority of Tregs from healthy individuals had an expression profile as would be expected in normally functioning cells. Less than three percent of patients with Crohn's disease belonged to this group, while the majority (almost 60 percent) showed a significantly altered expression profile, as is associated with the activation of proinflammatory signalling pathways.
"The Tregs from Crohn's disease patients react unusually to the tumour necrosis factor alpha (TNF-a) messenger substance that is secreted by the surrounding immune cells. We were able to identify ten different genes that are specifically upregulated only in Tregs from Crohn’s disease patients," explains the lead author of the study, which was published in the renowned journal Gastroenterology in April 2024.2) "And we succeeded in finding an active substance that can be used to reverse this development."
Epigenetically active drug reprogrammes Tregs
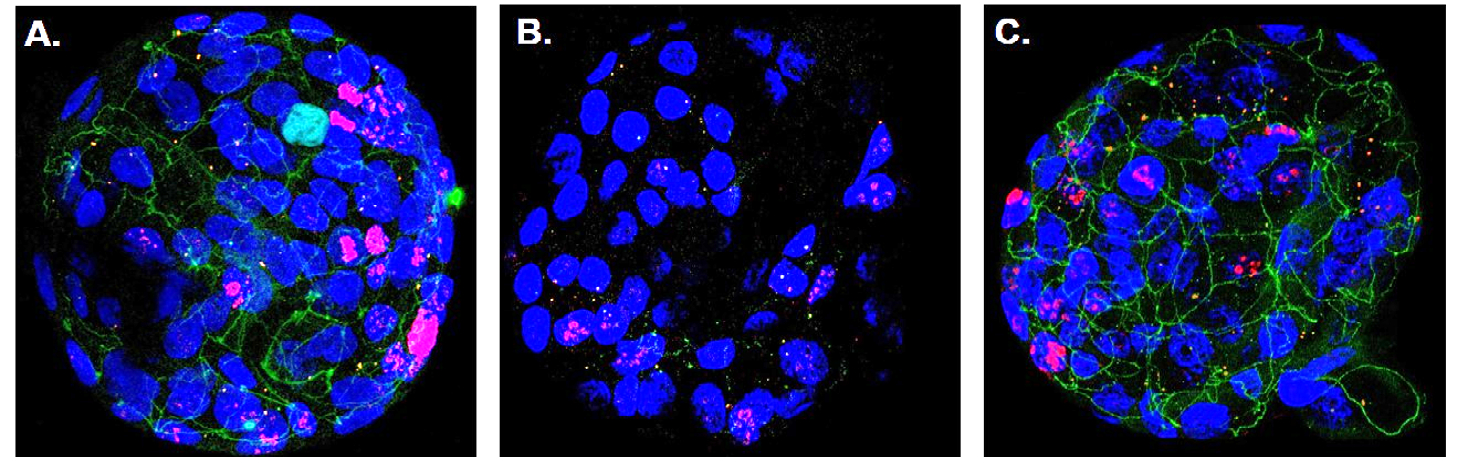 Immunofluorescence image of human colon organoids with blue glowing cell nuclei. When co-cultivated with healthy Tregs, tight junctions (green) are formed, which hold the epithelial cells tightly together (A). In the presence of proinflammatory Tregs, there are hardly any tight junctions present, resulting in larger intercellular spaces (B). Cultivation with proinflammatory Tregs and additional administration of vorinostat again leads to the formation of tight junctions (C). © Gastroenterology, Elsevier Inc.
Immunofluorescence image of human colon organoids with blue glowing cell nuclei. When co-cultivated with healthy Tregs, tight junctions (green) are formed, which hold the epithelial cells tightly together (A). In the presence of proinflammatory Tregs, there are hardly any tight junctions present, resulting in larger intercellular spaces (B). Cultivation with proinflammatory Tregs and additional administration of vorinostat again leads to the formation of tight junctions (C). © Gastroenterology, Elsevier Inc.The researchers used a bioinformatic analysis programme in order to reverse the dysregulation. The programme searches a drug database for substances that alter the transcription of relevant genes based on gene expression differences between healthy and diseased individuals. In so doing, they came across vorinostat, a drug approved for sale in the USA. Vorinostat influences the epigenetic mechanisms in the cell nucleus by inhibiting the activity of so-called histone deacetylases (HDACs).3) Cell culture experiments showed that vorinostat drastically reduces the expression of proinflammatory genes in Tregs, so that they are once again able to suppress the proliferation of other T lymphocytes.
This effect was also confirmed in experiments with human colon organoids. A strong loss of the contact points between the epithelial cells, the tight junctions, was observed when the organ-like microstructures were cultivated together with proinflammatory Tregs. The permeability of the epithelial layer increased significantly, a phenomenon that is also observed in the intestinal mucosa of Crohn's disease patients. This could be prevented by the addition of vorinostat.
"The proinflammatory Tregs we discovered and their reprogramming with the help of vorinostat could potentially open up new possibilities for treating IBDs," explains Kosinsky. There were already indications that the drug, which was previously only approved for the treatment of advanced cutaneous T-cell lymphoma, had an anti-inflammatory effect.3) This research is now uncovering important molecular principles.
The newly established working group in Stuttgart was primarily responsible for the bioinformatic analyses, while Kosinsky carried out the underlying laboratory work with the help of the Mayo Clinic team in the USA. "We have now established a comprehensive modelling system on site, which we can also use to investigate the influence of other immune cells on the permeability of the intestinal epithelium," says the group leader. "We also want to test other HDAC inhibitors that have fewer side effects." In this context, she emphasises that all research at the Bosch Health Campus, which includes the Robert Bosch Centre for Tumour Diseases, the Robert Bosch Hospital and other research institutes, is closely aligned with the clinic’s needs.